If you’re modeling protein transitions using interpolation techniques like ARAP (As-Rigid-As-Possible), one of the first hurdles is also the most essential: cleaning your input structures. This might seem like a straightforward step, but minor details — unconnected chains, waters, ligands — may quietly break your pipeline and throw hard-to-decipher errors.
This post explores a pain point shared by many molecular modelers: ensuring that protein structures are properly aligned, connected, and cleaned before running any structural interpolation. We’ll walk through a quick example using SAMSON’s built-in tools and demonstrate how to avoid structural issues that can make your analysis fail or produce misleading results.
Why protein preparation matters
When generating an interpolated path between two conformations of a protein — say, using the ARAP Interpolator in SAMSON — the tool maps and matches geometries of atoms across structures. If your input conformations have water molecules, ions, ligands or additional chains, the algorithm may not interpret them as matching structures, and you could end up with the following error:
Cannot proceed because the structure does not make one connected component.
This simply means something extraneous is breaking connectivity in the molecular graph. Here’s how to fix it in seconds.
Preparing the Diphtheria Toxin example
Let’s say you’re using two conformations of the Diphtheria Toxin protein: 1DDT and 1MDT. After fetching those structures in SAMSON via Home > Fetch, you’ll see that 1MDT has two chains. For a clean interpolation between just chain A of both structures, follow these key steps:
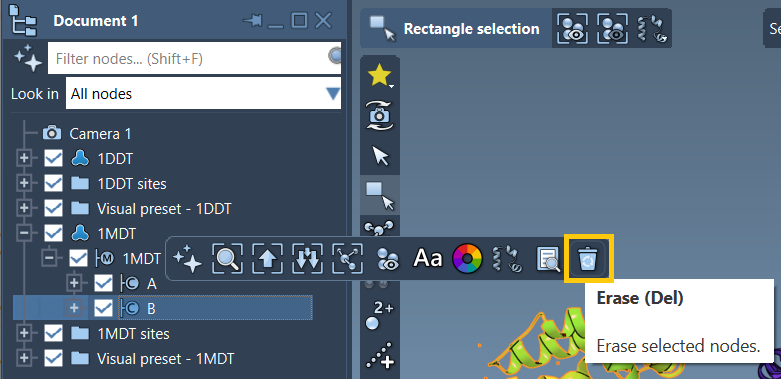
1. Delete chain B
Once you’ve loaded 1MDT, expand its node in the Document view to see both chains. Select chain B and delete it using the Del key or the erase icon from the toolbar popup. This avoids deceptively silent mismatches when chains are not structurally equivalent.
2. Clean both structures
Click on Home > Prepare for each structure. This tool automatically removes:
- Alternate atom locations
- Water molecules
- Ions
- Ligands
This short step makes sure each structure is a connected, clean protein backbone ready for further analysis.
Don’t skip this step — it can save hours later
Preparation is not just about tidying up. If you neglect to clean the structures, downstream tools like the ARAP Interpolator may produce errors—not because they are buggy, but because your inputs include non-equivalent structural parts.
It’s worth noting that SAMSON provides a guided tutorial for preparing protein structures. This can be useful if your protein system is more complex or includes cofactors or small molecule ligands.
Once you’ve cleaned and validated your structures, you can proceed confidently to build conformations and generate interpolated pathways—whether for use in umbrella sampling, steered MD, or dimensionality reduction.
Learn more in the original documentation.
SAMSON and all SAMSON Extensions are free for non-commercial use. You can download SAMSON at https://www.samson-connect.net.





