When designing new molecular analogues, chemists often wonder: Which part of the molecule should I modify to explore new activity profiles? In early-stage drug design, this is one of the most frequent roadblocks. If you’re interested in systematic molecular modification, one powerful yet accessible approach is pattern-based analogue scanning, using SMARTS patterns.
In the context of SAMSON’s SMILES Manager, you can define specific molecular patterns and make selective substitutions to generate novel analogues simply and interactively. This post focuses specifically on the step of defining a target pattern in your molecule — a key decision that shapes your analogue design strategy.
Why SMARTS Patterns Matter
SMARTS (SMiles ARbitrary Target Specification) is a language that lets you specify substructural patterns in molecules. By entering a SMARTS code, you tell SAMSON what part of the molecule you’re interested in modifying. For example, if you use [cH], you’re selecting an aromatic carbon that bears one hydrogen — in other words, a modifiable spot on a benzene-like ring.
Selecting the Pattern: An Example
Let’s assume you’ve already loaded a molecule into SAMSON via its SMILES code or selected it in your document. The next step is critical: deciding where to explore changes.
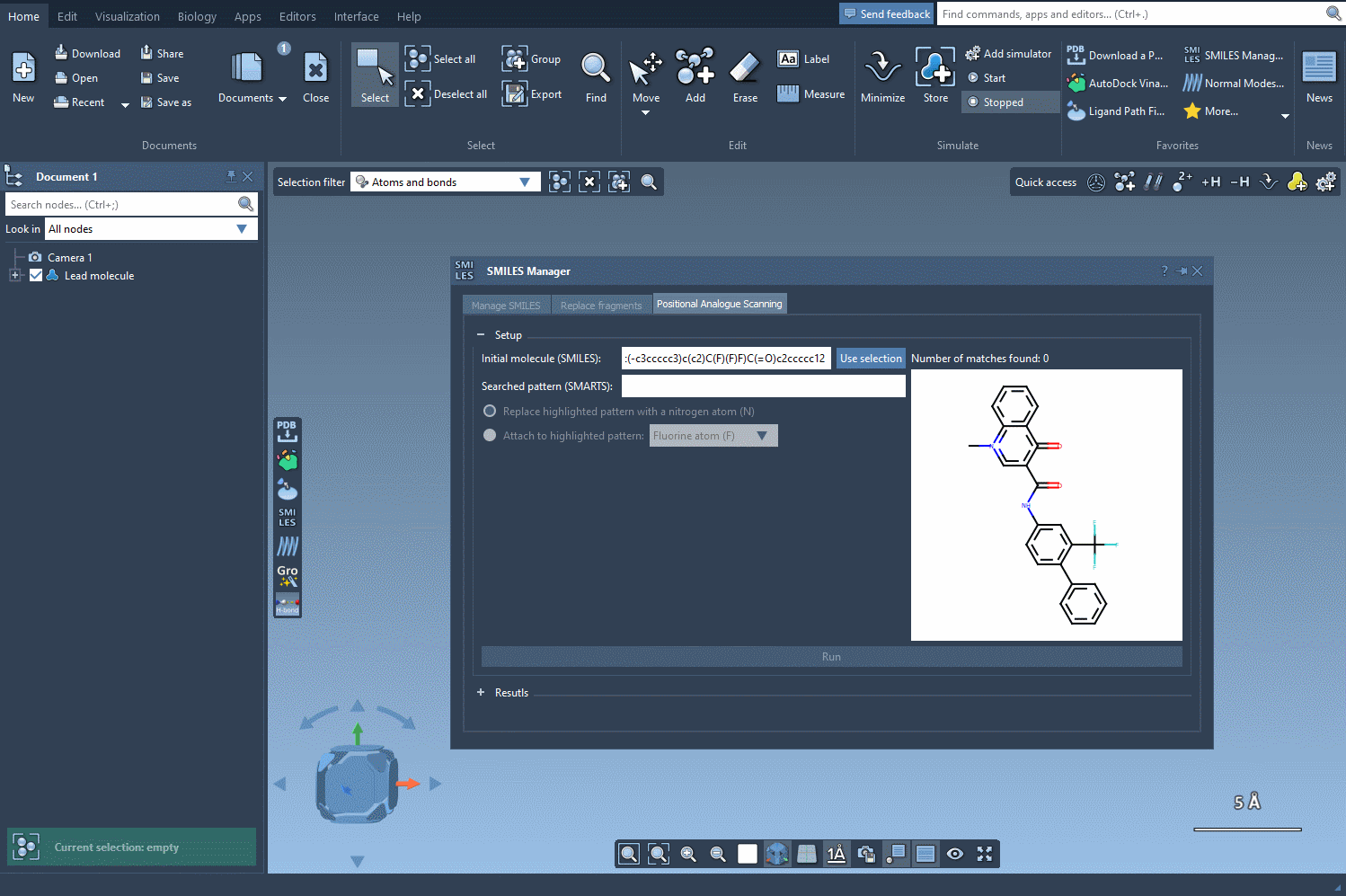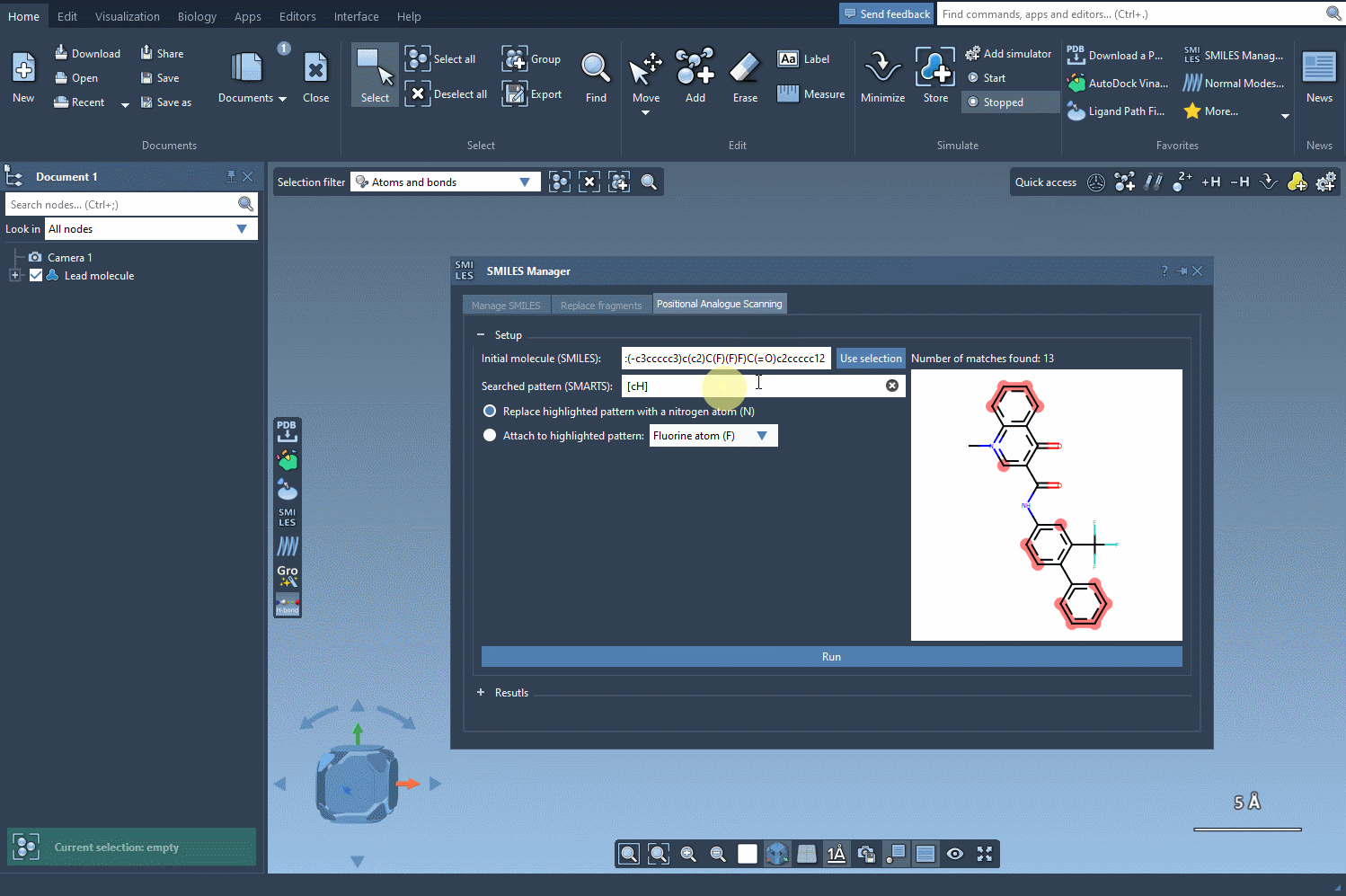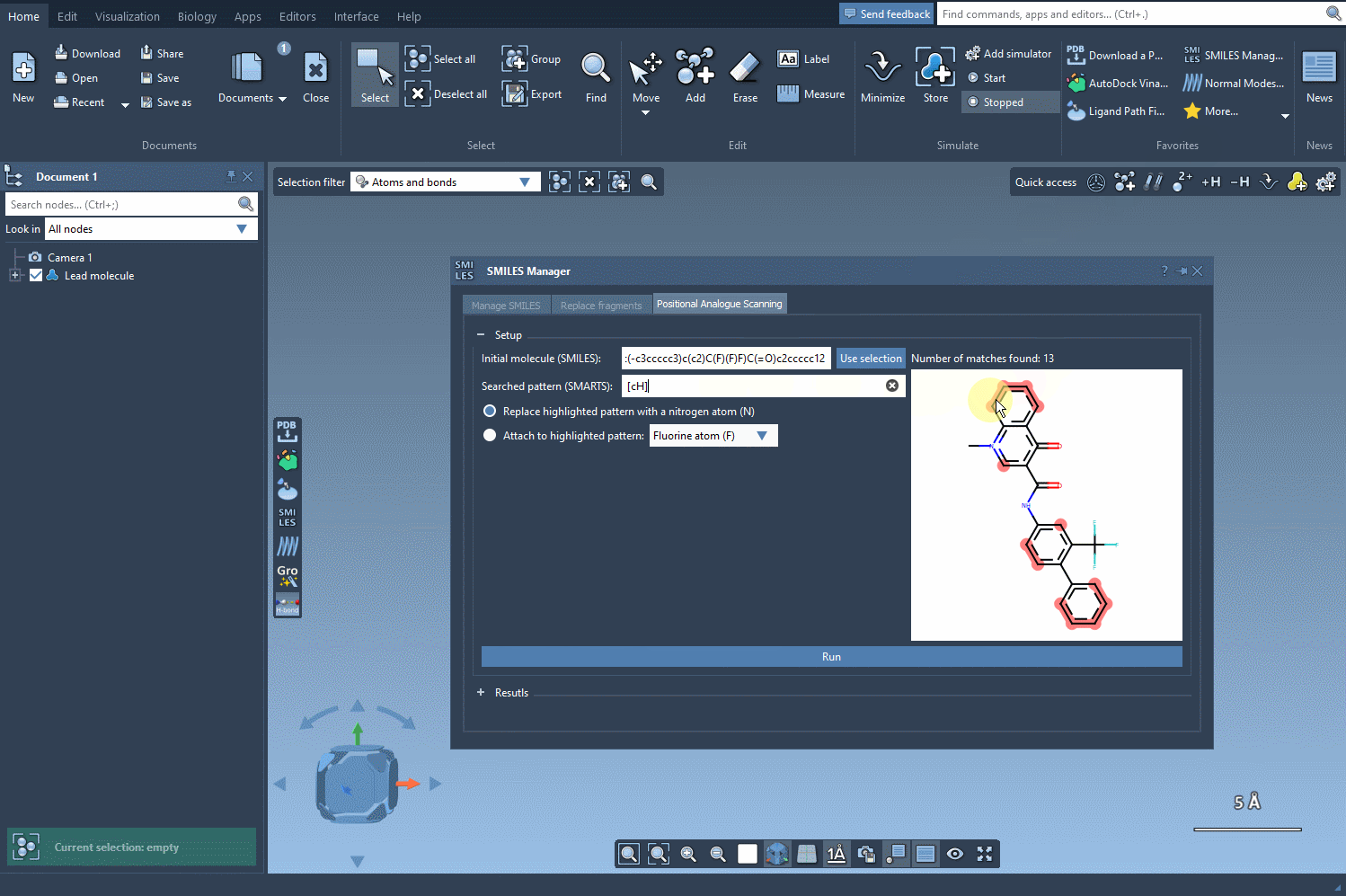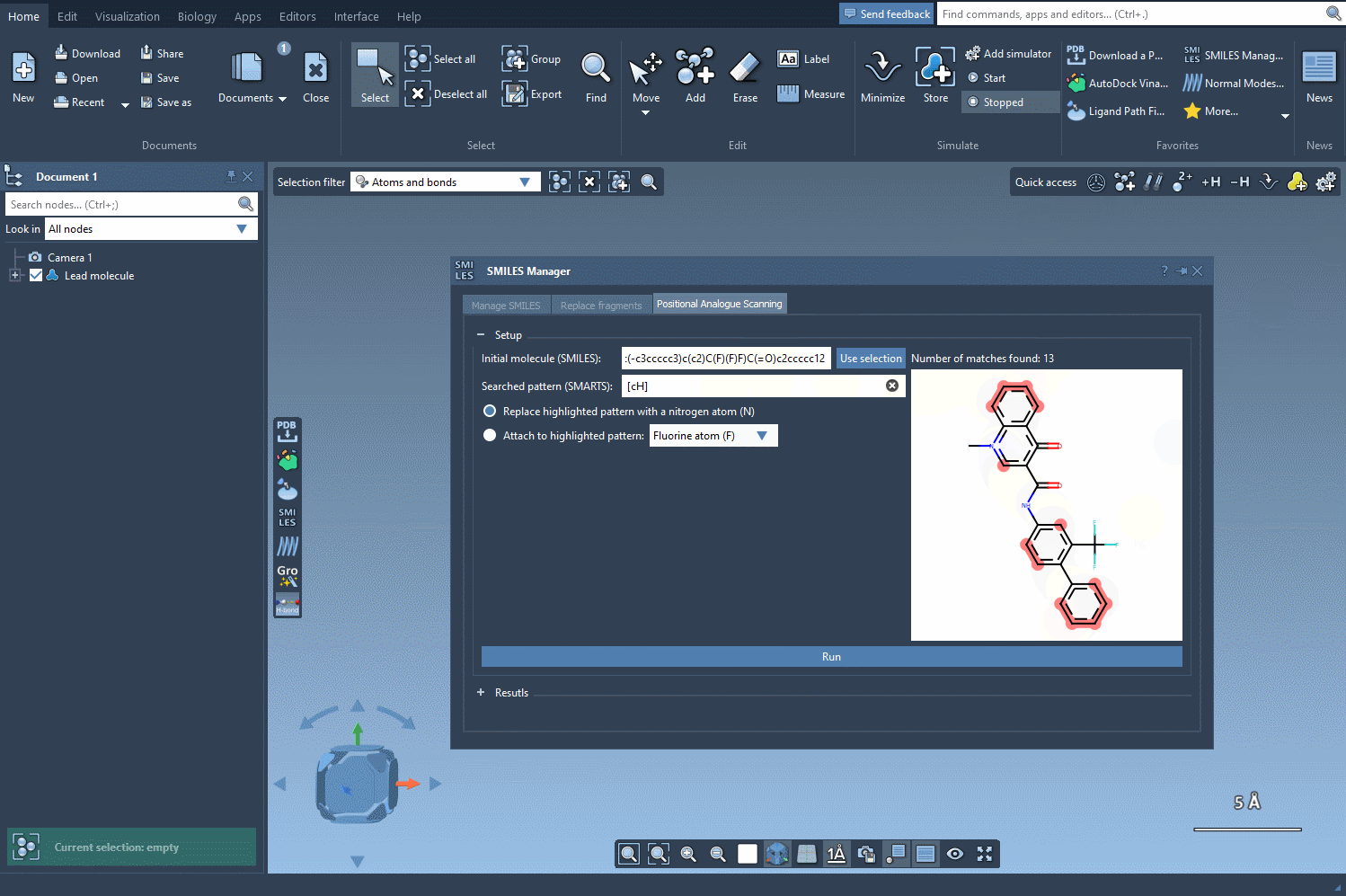
For instance, if your goal is to understand how modifications to an aromatic ring affect binding affinity or solubility, you might enter [cH] in the SMARTS pattern field of the SMILES Manager. This indicates that you’re targeting aromatic hydrogens, a common strategy in medicinal chemistry designs.
Once entered, SAMSON will visually highlight all matching atoms in real time. This feedback helps you quickly confirm whether your SMARTS expression targets the intended region.
What to Modify: Attach or Replace?
After identifying the atoms matching your pattern, you can choose to either replace them (e.g., with N or OH groups) or attach new elements (e.g., F, CH3). These small changes can yield large insights into structure–activity relationships (SAR).
This flexibility gives you control over how aggressively you alter your molecule — whether you prefer bioisosteric replacements or subtle fine-tuning. And because the SMARTS syntax is so expressive, you can move beyond basic modifications and create tailored libraries of analogues targeting specific structural motifs.
Visual, Iterative Design
The interactive nature of this process makes it easy to explore different patterns quickly. You could start with a simple [cH], then experiment with broader or more restrictive patterns depending on your SAR hypothesis. Each modification updates on screen, so you remain in control throughout the analogue generation workflow.
Conclusion
Defining the right SMARTS pattern is often the linchpin of efficient analogue generation. It lets you apply medicinal chemistry reasoning to molecular design in a systematic, visual way. Whether you’re exploring halogen substitutions, ring modifications, or heteroatom replacements, SMARTS-based pattern definition gives you a fast path from hypothesis to data.
To learn more about analogue generation workflows in SAMSON, including how to run docking simulations and study interactions, visit the full tutorial page here.
SAMSON and all SAMSON Extensions are free for non-commercial use. Get SAMSON at https://www.samson-connect.net.





