When modeling molecular transitions, setting up your system properly is as important as the results you want to obtain. Protein structures from the Protein Data Bank (PDB) are often not ready to be used directly in simulations or path interpolations. They typically contain water molecules, ions, ligands, alternate locations, or even disconnected fragments. If not handled correctly, these issues might lead to errors or biologically irrelevant outputs when running interpolation or dynamics calculations.
One common error you might encounter when running an As-Rigid-As-Possible (ARAP) interpolation in SAMSON is:
“Cannot proceed because the structure does not make one connected component”
What does that mean in practice? It simply means the system is disconnected in space — maybe there’s a ligand floating in the structure, or some water molecules are not bonded to anything. And that blocks the computation. Thankfully, SAMSON provides a quick way to fix this.
Cleaning Structures in SAMSON
Before running your path interpolation or simulation, go to:
Home > Prepare
This tool automatically removes:
- Alternate locations
- Water molecules
- Ligands
- Ions
By doing this, you ensure your structure is a single, connected macromolecule — optimal for geometry-based interpolations and other simulations.
An Example: Cleaning Diphtheria Toxin Structures
Let’s say you’re working with two protein conformations of the diphtheria toxin: 1DDT and 1MDT. Both contain chains A and B, but you only need chain A for your study.
In the Document view of SAMSON:
- Expand
1MDT - Select and delete chain
B
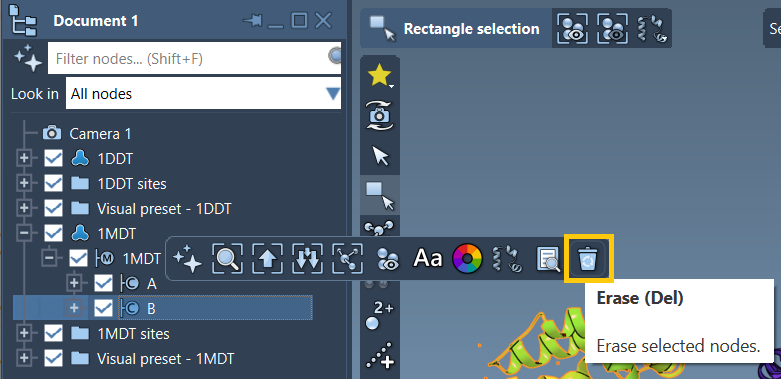
After focusing on the correct chain, use Home > Prepare to clean both 1DDT and 1MDT.
This might seem like a small step, but it’s foundational. A clean, connected structure gives better interpolation results and prevents workflow interruptions by errors.
Why This Matters
Many molecular modelers spend unnecessary time debugging workflows because their input structures were not properly cleaned. Hydrogens in experimental structures may be missing entirely; ligands may not be relevant for your transition; and water or ions can break assumptions in many algorithms.
Proper structure preparation serves several purposes:
- Ensures consistent atom matching
- Prevents interpolation and simulation failures
- Leads to more biologically meaningful results
You can also refer to SAMSON’s Protein Preparation & Validation tutorial if you’re unsure whether a structure is ready.
While often overlooked, this step is crucial — and it only takes a few seconds, thanks to SAMSON’s preparation tools.
Learn More
To study protein transition pathways or learn about ARAP interpolation, visit the full guide at this documentation page.
SAMSON and all SAMSON Extensions are free for non-commercial use. You can download SAMSON here.





