When preparing to model protein transitions—such as conformational changes or reaction pathways—many molecular modelers face a frustrating issue: their interpolation algorithm fails to run, or produces unrealistic outputs. A frequent cause? Poorly prepared structures.
If you’re using the As-Rigid-As-Possible (ARAP) Interpolator in SAMSON to generate a morphing trajectory between two protein structures, there’s a small but critical step that can make or break your simulation: cleaning your input conformations.
Why Clean Protein Structures?
Real-world PDB files often contain features that are problematic for interpolation methods:
- Alternate atom locations
- Unresolved segments
- Water molecules, ions, and ligands
While these elements might be useful in some simulations, they introduce disconnected components that confuse graph-based interpolation algorithms like ARAP. The result? An error message like:
“Cannot proceed because the structure does not make one connected component.”
This occurs because the structure includes fragments that aren’t covalently connected, making it ambiguous how to create continuous, physically meaningful deformation paths.
How to Properly Prepare Your Protein Structures in SAMSON
Fortunately, SAMSON offers a simple built-in solution. Before you begin generating conformations for interpolation, follow these steps:
- Go to Home > Prepare.
- This tool will clean each structure by removing alternate locations, water molecules, ligands, and ions.
- Repeat this process for both your starting and goal structures.
Once done, you will have structures that are topologically clean and ready for interpolation.
A Quick Check: One Connected Component
After running Prepare, ensure that your molecule is a single connected structure. In SAMSON’s Document view, expand the structure hierarchy and verify that no unwanted chains or small fragments remain. You may manually delete anything that’s not part of the main protein chain of interest.
This not only prevents computational errors but also ensures that the ARAP algorithm produces realistic intermediate conformations between your protein states.
Before & After: Make It Visual
Here’s an example from the ARAP Interpolation tutorial, where chain B (unneeded) in the 1MDT structure is deleted prior to interpolation:
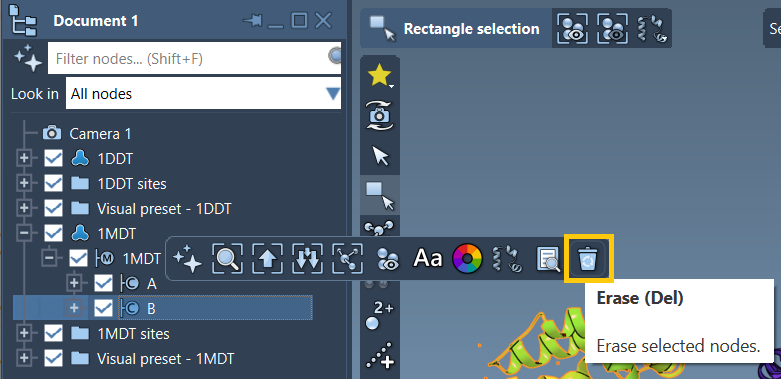
By removing redundant chains or ligand components and cleaning the structure, the path interpolation becomes smooth and visually meaningful.
When in Doubt, Refer to the Tutorial
If you’re unsure how best to prepare a particular protein, refer to the official guide: Protein Preparation & Validation.
To learn more about generating paths between protein structures using ARAP interpolation, visit the full documentation page: ARAP Interpolation for Protein Structures – Documentation.
SAMSON and all SAMSON Extensions are free for non-commercial use. You can download SAMSON at https://www.samson-connect.net.





